Module: Baseline Metrics#
When assessing the quality of a segmentation, the first thing one usually looks at are summary metrics such as the number of segmented cells, number of transcripts/genes per cell, the percentage of unassigned transcripts, the transcript density, and a variety of morphological features.
The baseline (bl) module contains several metrics that can help you to assess the quality of your segmentation. The methods all return their corresponding values or dataframes, and also write them into the spatialdata object.
To follow along with this tutorial, you can download the data from here.
[1]:
%load_ext autoreload
%autoreload 2
[2]:
import math
import matplotlib.pyplot as plt
import spatialdata as sd
import segtraq
sdata = sd.read_zarr("../../data/xenium_5K_data/proseg2.zarr")
# putting the spatialdata object into a SegTraQ constructor
# this has the advantage that we only need to set keywords like cell IDs or transcript IDs once
st = segtraq.SegTraQ(
sdata,
images_key=None,
tables_area_key=None,
points_background_id=0,
tables_centroid_x_key="centroid_x",
tables_centroid_y_key="centroid_y",
)
/g/huber/users/meyerben/notebooks/spatial_transcriptomics/SegTraQ/.venv/lib/python3.13/site-packages/spatialdata/_core/query/relational_query.py:531: FutureWarning: functools.partial will be a method descriptor in future Python versions; wrap it in enum.member() if you want to preserve the old behavior
left = partial(_left_join_spatialelement_table)
/g/huber/users/meyerben/notebooks/spatial_transcriptomics/SegTraQ/.venv/lib/python3.13/site-packages/spatialdata/_core/query/relational_query.py:532: FutureWarning: functools.partial will be a method descriptor in future Python versions; wrap it in enum.member() if you want to preserve the old behavior
left_exclusive = partial(_left_exclusive_join_spatialelement_table)
/g/huber/users/meyerben/notebooks/spatial_transcriptomics/SegTraQ/.venv/lib/python3.13/site-packages/spatialdata/_core/query/relational_query.py:533: FutureWarning: functools.partial will be a method descriptor in future Python versions; wrap it in enum.member() if you want to preserve the old behavior
inner = partial(_inner_join_spatialelement_table)
/g/huber/users/meyerben/notebooks/spatial_transcriptomics/SegTraQ/.venv/lib/python3.13/site-packages/spatialdata/_core/query/relational_query.py:534: FutureWarning: functools.partial will be a method descriptor in future Python versions; wrap it in enum.member() if you want to preserve the old behavior
right = partial(_right_join_spatialelement_table)
/g/huber/users/meyerben/notebooks/spatial_transcriptomics/SegTraQ/.venv/lib/python3.13/site-packages/spatialdata/_core/query/relational_query.py:535: FutureWarning: functools.partial will be a method descriptor in future Python versions; wrap it in enum.member() if you want to preserve the old behavior
right_exclusive = partial(_right_exclusive_join_spatialelement_table)
no parent found for <ome_zarr.reader.Label object at 0x7f17072adfd0>: None
no parent found for <ome_zarr.reader.Label object at 0x7f17072c6350>: None
no parent found for <ome_zarr.reader.Label object at 0x7f17072c6710>: None
no parent found for <ome_zarr.reader.Label object at 0x7f17072b3230>: None
no parent found for <ome_zarr.reader.Label object at 0x7f17075536f0>: None
no parent found for <ome_zarr.reader.Label object at 0x7f17072ccef0>: None
/g/huber/users/meyerben/notebooks/spatial_transcriptomics/SegTraQ/src/segtraq/SegTraQ.py:130: UserWarning: Missing 4 cell IDs in shapes: [np.int64(11273), np.int64(10307), np.int64(10308), np.int64(6870)]... These cells are present in tables, but not in shapes. This might lead to inconsistencies in the spatialdata object.
validate_spatialdata(
/g/huber/users/meyerben/notebooks/spatial_transcriptomics/SegTraQ/src/segtraq/utils.py:1255: UserWarning: Duplicate IDs detected in index 'cell_id' for shapes 'nucleus_boundaries'. Resetting and renaming index to `segtraq_id` to ensure uniqueness.
nucleus_shapes = _ensure_index(
/g/huber/users/meyerben/notebooks/spatial_transcriptomics/SegTraQ/src/segtraq/SegTraQ.py:130: RuntimeWarning: No area column specified for tables. Area will be automatically computed from shapes.
validate_spatialdata(
To get a first impression of the quality of the data, we can check how many cells there are in the data. We can also check how many transcripts were measured in total, how many genes they map to, and how many transcripts were not assigned to a cell.
[3]:
st.bl.num_cells()
[3]:
17885
[4]:
st.bl.num_transcripts()
[4]:
2189801
[5]:
st.bl.num_genes()
[5]:
5095
[6]:
st.bl.perc_unassigned_transcripts()
[6]:
2.0661238167303786
Next, let’s see how many transcripts were detected per cell. We can do this using the transcripts_per_cell() method.
[7]:
transcripts_per_cell = st.bl.transcripts_per_cell()
transcripts_per_cell.head()
[7]:
| cell_id | transcript_count | |
|---|---|---|
| 0 | 3446 | 2345 |
| 1 | 1097 | 1777 |
| 2 | 2722 | 1458 |
| 3 | 2006 | 1407 |
| 4 | 2100 | 1314 |
We can plot the median and distribution of this to see how the number of transcripts differs across cells.
[8]:
# plotting the number of transcripts per cell
plt.figure(figsize=(10, 6))
plt.hist(transcripts_per_cell["transcript_count"], bins=100)
# adding a line for the median
plt.axvline(
transcripts_per_cell["transcript_count"].median(),
color="black",
linestyle="dashed",
linewidth=1,
)
plt.text(
transcripts_per_cell["transcript_count"].median() + 5,
50,
f"Median: {transcripts_per_cell['transcript_count'].median():.2f}",
color="black",
)
# adding labels and title
plt.xlabel("Number of Transcripts")
plt.ylabel("Count")
plt.title("Distribution of Transcripts per Cell")
plt.show()
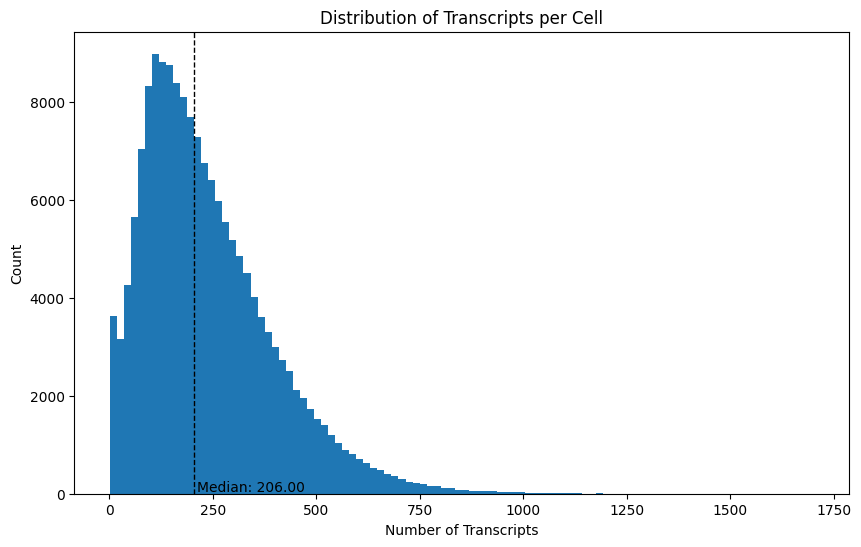
We can also do the same thing for the number of genes per cell, since there are often multiple transcripts measured per gene.
[9]:
genes_per_cell = st.bl.genes_per_cell()
genes_per_cell.head()
[9]:
| cell_id | gene_count | |
|---|---|---|
| 0 | 1 | 389 |
| 1 | 2 | 45 |
| 2 | 3 | 5 |
| 3 | 4 | 120 |
| 4 | 5 | 41 |
[10]:
# plotting the number of genes per cell
plt.figure(figsize=(10, 6))
plt.hist(genes_per_cell["gene_count"], bins=100)
# adding a line for the median
plt.axvline(
genes_per_cell["gene_count"].median(),
color="black",
linestyle="dashed",
linewidth=1,
)
plt.text(
genes_per_cell["gene_count"].median() + 5,
50,
f"Median: {genes_per_cell['gene_count'].median():.2f}",
color="black",
)
# adding labels and title
plt.xlabel("Number of Genes")
plt.ylabel("Count")
plt.title("Distribution of Genes per Cell")
plt.show()
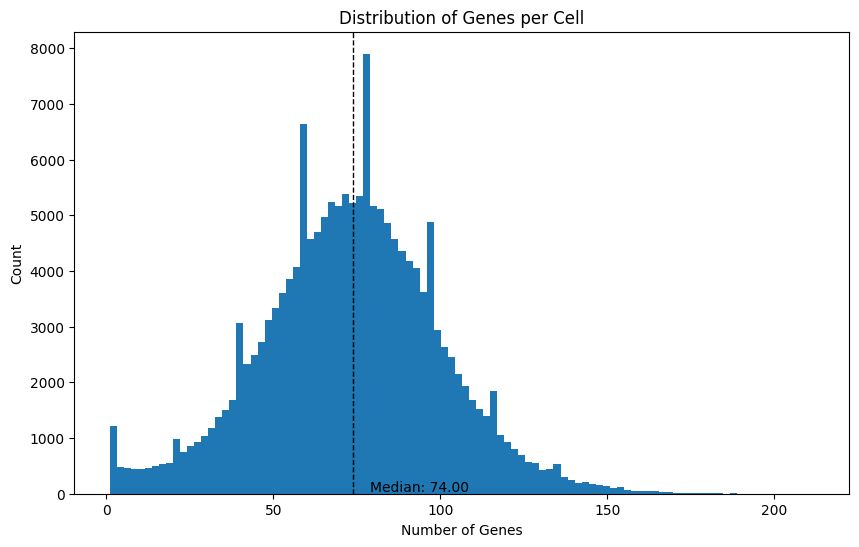
Next to the number of transcripts per cell, we can also investigate the transcript density, which is computed as the number of transcripts divided by the cell area. Note that the background does not appear in this data frame.
[11]:
transcript_density = st.bl.transcript_density()
transcript_density.head()
[11]:
| cell_id | transcript_density | |
|---|---|---|
| 0 | 3446 | 3.674109 |
| 1 | 1097 | 4.498734 |
| 2 | 2722 | 5.559581 |
| 3 | 2006 | 4.456057 |
| 4 | 2100 | 3.913626 |
[12]:
x = transcript_density["transcript_density"].dropna()
p99 = x.quantile(0.99)
x_clip = x[x <= p99]
plt.figure(figsize=(10, 6))
plt.hist(x_clip, bins=100)
# median from full distribution
med = x.median()
plt.axvline(med, color="black", linestyle="dashed", linewidth=1)
plt.text(
med + 0.05,
plt.ylim()[1] * 0.9,
f"Median: {med:.2f}",
color="black",
)
# adding labels and title
plt.xlabel("Transcript Density (transcripts per area)")
plt.ylabel("Count")
plt.title("Distribution of Transcript Density per Cell")
plt.show()
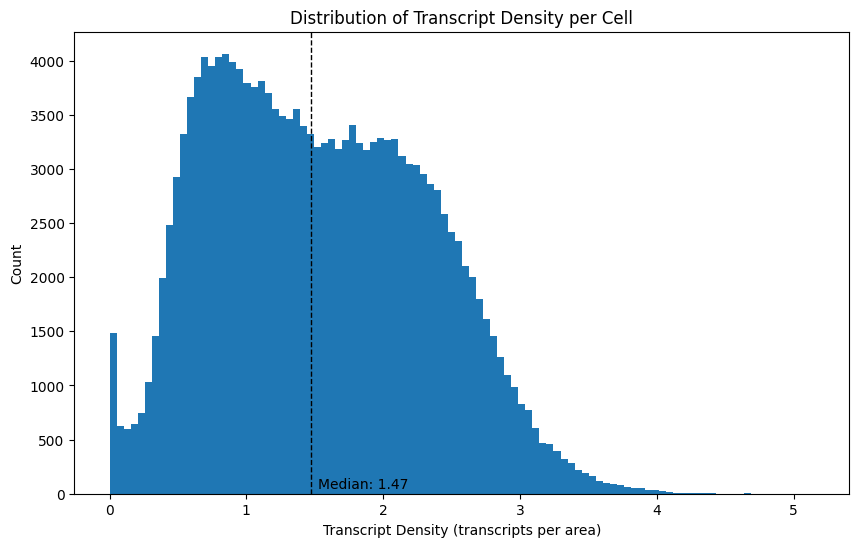
We can also compute the mean number of transcripts per detected gene per cell, which is computed by averaging per-gene transcript counts across genes observed in each cell. Note that only detected genes are considered and background transcripts are excluded.
[13]:
mean_transcripts_per_gene_per_cell = st.bl.mean_transcripts_per_gene_per_cell()
mean_transcripts_per_gene_per_cell.head()
[13]:
| cell_id | mean_transcripts_per_gene | |
|---|---|---|
| 0 | 1 | 1.375321 |
| 1 | 2 | 1.066667 |
| 2 | 3 | 1.200000 |
| 3 | 4 | 1.250000 |
| 4 | 5 | 1.146341 |
[14]:
x = mean_transcripts_per_gene_per_cell["mean_transcripts_per_gene"].dropna()
p99 = x.quantile(0.99)
x_clip = x[x <= p99]
plt.figure(figsize=(10, 6))
plt.hist(x_clip, bins=100)
# median from full distribution
med = x.median()
plt.axvline(med, color="black", linestyle="dashed", linewidth=1)
plt.text(
med + 0.05,
plt.ylim()[1] * 0.9,
f"Median: {med:.2f}",
color="black",
)
# adding labels and title
plt.xlabel("Transcript Density (transcripts per area)")
plt.ylabel("Count")
plt.title("Distribution of Transcript Density per Cell")
plt.show()
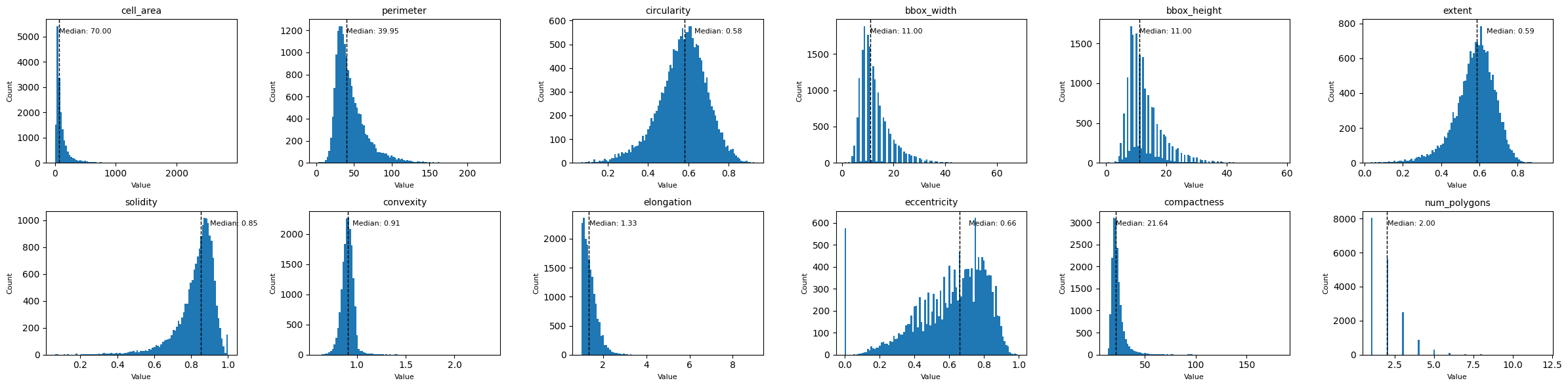
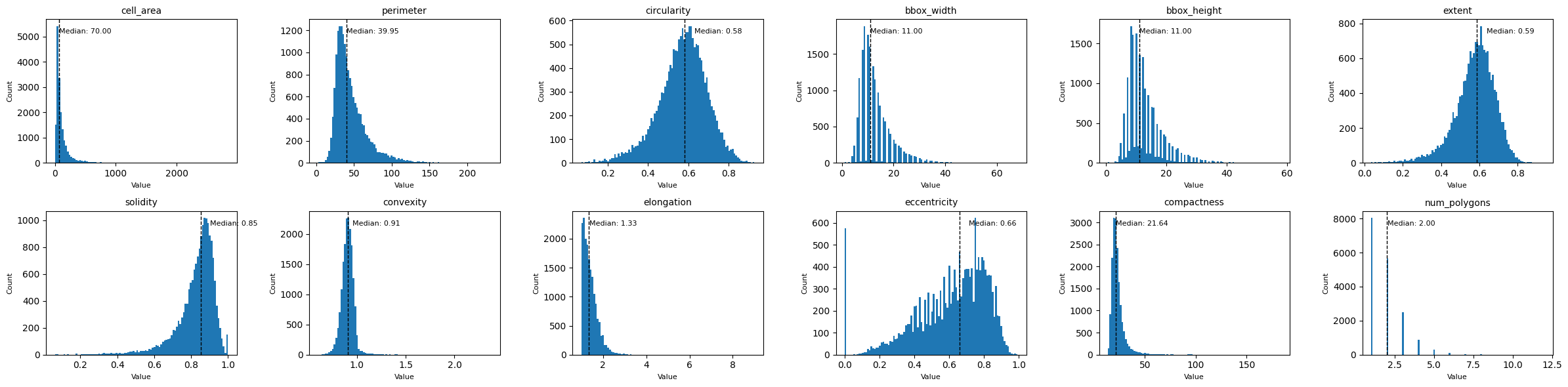
Finally, let’s look at some morphological features, such as the cell area, circularity, elongation, etc. We can get those with the function morphological_features(). If you only want to compute certain features, you can select them with the features_to_compute argument. This can drastically reduce the runtime, as especially the features elongation and eccentricity can take a while to compute.
[15]:
morphological_features = st.bl.morphological_features()
morphological_features.head()
[15]:
| cell_id | cell_area | perimeter | circularity | bbox_width | bbox_height | extent | solidity | convexity | elongation | eccentricity | compactness | num_polygons | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 223.75 | 77.291274 | 0.470665 | 13.0 | 28.0 | 0.614698 | 0.874023 | 0.867660 | 2.153846 | 0.885685 | 26.699178 | 3 |
| 1 | 2 | 48.50 | 28.825554 | 0.733494 | 9.0 | 9.0 | 0.598765 | 0.906542 | 0.955507 | 1.090909 | 0.399653 | 17.132217 | 1 |
| 2 | 3 | 35.00 | 27.756656 | 0.570878 | 9.0 | 7.0 | 0.555556 | 0.853659 | 0.914635 | 1.368421 | 0.682625 | 22.012341 | 1 |
| 3 | 4 | 75.25 | 42.007644 | 0.535870 | 12.0 | 11.0 | 0.570076 | 0.817935 | 0.865989 | 1.016854 | 0.181313 | 23.450394 | 3 |
| 4 | 5 | 92.50 | 44.342251 | 0.591175 | 14.0 | 11.0 | 0.600649 | 0.864486 | 0.863819 | 1.119565 | 0.449652 | 21.256597 | 1 |
Let’s plot all of the distributions in one plot using subplots.
[16]:
# exclude 'cell_id' from the features to plot
features = [f for f in morphological_features.columns if f != "cell_id"]
# define grid layout
num_features = len(features)
cols = 6
rows = math.ceil(num_features / cols)
# create subplots
fig, axes = plt.subplots(rows, cols, figsize=(cols * 4, rows * 3))
axes = axes.flatten()
for i, feature in enumerate(features):
ax = axes[i]
data = morphological_features[feature]
ax.hist(data, bins=100)
median_val = data.median()
ax.axvline(median_val, color="black", linestyle="dashed", linewidth=1)
ax.text(
median_val + 0.05,
ax.get_ylim()[1] * 0.9,
f"Median: {median_val:.2f}",
color="black",
fontsize=8,
)
ax.set_title(feature, fontsize=10)
ax.set_xlabel("Value", fontsize=8)
ax.set_ylabel("Count", fontsize=8)
# hide unused subplots
for j in range(i + 1, len(axes)):
fig.delaxes(axes[j])
fig.tight_layout()
plt.show()
Instead of measures per cell, we can also compute some metrics per gene. For example, we can see the percentage of how often a gene was not assigned to any cell. This can help to detect potential biases in our segmentation.
[17]:
perc_unassigned_transcripts_per_gene = st.bl.perc_unassigned_transcripts_per_gene()
perc_unassigned_transcripts_per_gene.sort_values(by="perc_unassigned", ascending=False).head()
[17]:
| total | unassigned | perc_unassigned | |
|---|---|---|---|
| feature_name | |||
| HPV16-E7 | 1 | 1 | 100.000000 |
| CIDEC | 169 | 105 | 62.130178 |
| GHR | 118 | 69 | 58.474576 |
| PLIN1 | 240 | 137 | 57.083333 |
| SLC1A1 | 22 | 12 | 54.545455 |
In our example, most transcripts were assigned to a cell. However, if you detected that a large number of transcripts were unassigned, you could follow up with a gene set enrichment analysis (GSEA) to look for specific biases.
Finally, we can check the anndata object to verify that all of our metrics are stored in there.
[18]:
sdata.tables["table"]
[18]:
AnnData object with n_obs × n_vars = 17885 × 5095
obs: 'cell_id', 'centroid_x', 'centroid_y', 'centroid_z', 'volume', 'label_id', 'region', 'gene_count', 'transcript_count', 'transcript_density', 'mean_transcripts_per_gene', 'cell_area', 'perimeter', 'circularity', 'bbox_width', 'bbox_height', 'extent', 'solidity', 'convexity', 'elongation', 'eccentricity', 'compactness', 'num_polygons'
var: 'total', 'unassigned', 'perc_unassigned'
uns: 'spatialdata_attrs', 'num_cells', 'num_transcripts', 'num_genes', 'perc_unassigned_transcripts'
layers: 'X_estimated'
Alternatively, all bl metrics can be computed in one run via run_baseline.
[19]:
st.run_baseline()
sdata.tables["table"].obs.head()
[19]:
| cell_id | centroid_x | centroid_y | centroid_z | volume | label_id | region | gene_count | mean_transcripts_per_gene | transcript_count | ... | circularity | bbox_width | bbox_height | extent | solidity | convexity | elongation | eccentricity | compactness | num_polygons | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 154.66340 | 1053.59360 | -0.115346 | 1131.67400 | 1 | cell_boundaries | 389.0 | 1.375321 | 535.0 | ... | 0.470665 | 13.0 | 28.0 | 0.614698 | 0.874023 | 0.867660 | 2.153846 | 0.885685 | 26.699178 | 3.0 |
| 1 | 2 | 348.67285 | 287.59875 | 0.524985 | 256.04938 | 2 | cell_boundaries | 45.0 | 1.066667 | 48.0 | ... | 0.733494 | 9.0 | 9.0 | 0.598765 | 0.906542 | 0.955507 | 1.090909 | 0.399653 | 17.132217 | 1.0 |
| 2 | 3 | 720.20215 | 1218.87230 | 0.660745 | 148.57190 | 3 | cell_boundaries | 5.0 | 1.200000 | 6.0 | ... | 0.570878 | 9.0 | 7.0 | 0.555556 | 0.853659 | 0.914635 | 1.368421 | 0.682625 | 22.012341 | 1.0 |
| 3 | 4 | 877.44590 | 1707.41210 | -0.980020 | 233.92108 | 4 | cell_boundaries | 120.0 | 1.250000 | 150.0 | ... | 0.535870 | 12.0 | 11.0 | 0.570076 | 0.817935 | 0.865989 | 1.016854 | 0.181313 | 23.450394 | 3.0 |
| 4 | 5 | 1253.77540 | 1426.49510 | 0.859675 | 327.17487 | 5 | cell_boundaries | 41.0 | 1.146341 | 47.0 | ... | 0.591175 | 14.0 | 11.0 | 0.600649 | 0.864486 | 0.863819 | 1.119565 | 0.449652 | 21.256597 | 1.0 |
5 rows × 23 columns
[20]:
sdata.tables["table"].var.head()
[20]:
| total | unassigned | perc_unassigned | |
|---|---|---|---|
| gene_symbol | |||
| EEF1G | 29662 | 568 | 1.914908 |
| XBP1 | 24838 | 42 | 0.169096 |
| TIMP3 | 18879 | 74 | 0.391970 |
| RAB11FIP1 | 15609 | 24 | 0.153757 |
| H3F3B | 13492 | 368 | 2.727542 |
Session Info#
[21]:
print(sd.__version__) # spatialdata
0.7.2
